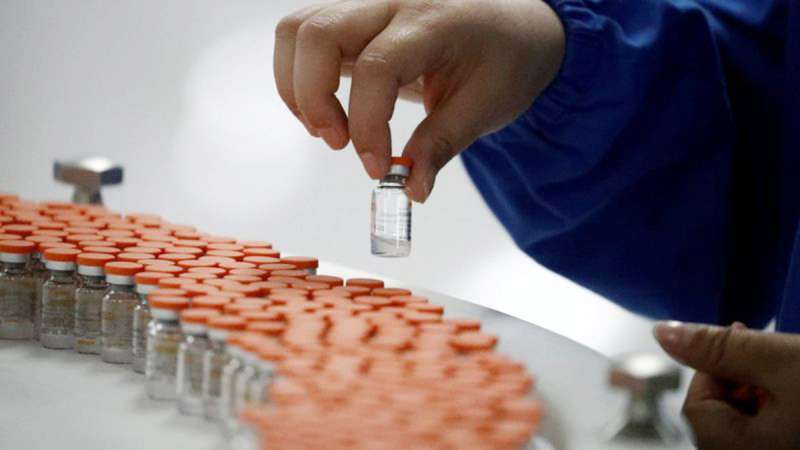
The Department of Health (DOH) has confirmed that the report involving the local pharmaceutical company Sinovac in the case of bribery or bribery will also be investigated by local experts.
This statement by the agency follows a report on the company’s bribery charges in 2003 and 2009.
“We are aware of what’s happening… so this will also form part of the work of the vaccine expert panel (VEP), they will assess the veracity of the report if it is true,” said Health Sec. Francisco Duque III.
“We cannot say if such reports have a questionable origin. So the thing to do is to validate, ”the secretary added.
An article in The Washington Post in the United States newspaper reported that the chief executive officer of Sinovac was involved in bribery. The court in China allegedly has records where the company official admitted that he paid more than $ 83,000 to a regulatory official from 2002 to 2011, in exchange for a quick process to certify their vaccines.
“Sinovac has acknowledged the bribery case involving its CEO, saying in regulatory filings that he cooperated with prosecutors and was not charged. The CEO said in testimony he could not refuse demands for money from a regulatory official, ”the article said.
The incident allegedly happened before the company started their clinical trial on the SARS and swine flu vaccine.
In 2017, the regulatory official who received the bribe was sentenced to 10 years in prison. The CEO of Sinovac, on the other hand, returned to his job after the case against him was not pursued.
The report also shows that 20 government officials and hospital administrators in China admitted to the court that from 2008 to 2016 they received bribes from some Sinovac employees.
In its last annual report released by the company in April, Sinovac said the Chinese authorities have not yet filed a lawsuit against their CEO. But they are said to be strictly enforcing policies today against corruption within the company.
“Sinovac’s Yin Weidong, now 56, was not charged and continues to oversee the company’s coronavirus-vaccine drive this year.”
SINOVAC: “FRONTRUNNER” IN COVID-19 VACCINE DEVELOPMENT
Sinovac is one of the leading pharmaceutical companies in the world today when it comes to the development of the COVID-19 vaccine.
Here in the Philippines, they were the first to receive approval from the vaccine expert panel in October. For now, they are still waiting for the result of the evaluation of the ethics review board to move their application for clinical trial to the Food and Drug Administration (FDA).
Unlike companies from the Americas, Europe, and Russia, Sinovac has not yet released a report on the effectiveness of their vaccine.
But in an article published in the well-known research journal The Lancet, it is said that the Sinovac vaccine was given an immediate immune response when studied in April and May.
“Taking safety, immunogenicity, and production capacity into account, the 3 μg dose of CoronaVac is the suggested dose for efficacy assessment in future phase 3 trials,” according to the study.
According to Sec. Duque, if the report can be proven true, they must weigh with the ethics review board and FDA whether the Sinovac vaccine will be allowed to enter the Philippines.
“If true, then its up for the VEP to include this in their final report and also FDA, so there is a Single Joint Ethics Review Board to see it and we will not fall victim to questionable transactions.” (with reports from The Washington Post)